Wir sind am 01.04. umgezogen! Unsere neue Adresse ist: Reimerstwiete 11, 20457 Hamburg
Well-established use applications for marketing authorization are becoming increasingly popular in the pharmaceutical industry, but this could soon come to an end with the future EU pharmaceutical package.
There are many ways to submit an application for marketing authorization for medicinal products in accordance with EU/2001/83. In addition to the comprehensive full application (according to Art. 8.3), the generic application (according to Art. 10.1) and the hybrid application (according to Art. 10.3), there is also a special form of marketing authorization application, the well-established use (WEU) application (according to Art. 10a). This eliminates the need to submit your own preclinical and clinical data in modules 4 and 5. Instead, the prerequisite for this is proof that the active substances of the medicinal product in question have already been in general medical use within the European Community for at least a decade and at the same time have a recognized efficacy and acceptable safety.
The well-established use application has various advantages and disadvantages depending on the perspective. From the point of view of the authorities and patient safety, a full application with clinical and preclinical studies is considered the gold standard. In contrast, the utilisation of well-established use application acknowledges the demand for medicinal products and historical treatment options. This swift and cost-effective process significantly contributes to ensuring a secure supply. As the applicant needs to consider both positive and negative literature data and engage in controversial discussions about missing information to demonstrate the effectiveness and safety of the drug, this regulatory application also ensures adequate patient safety.
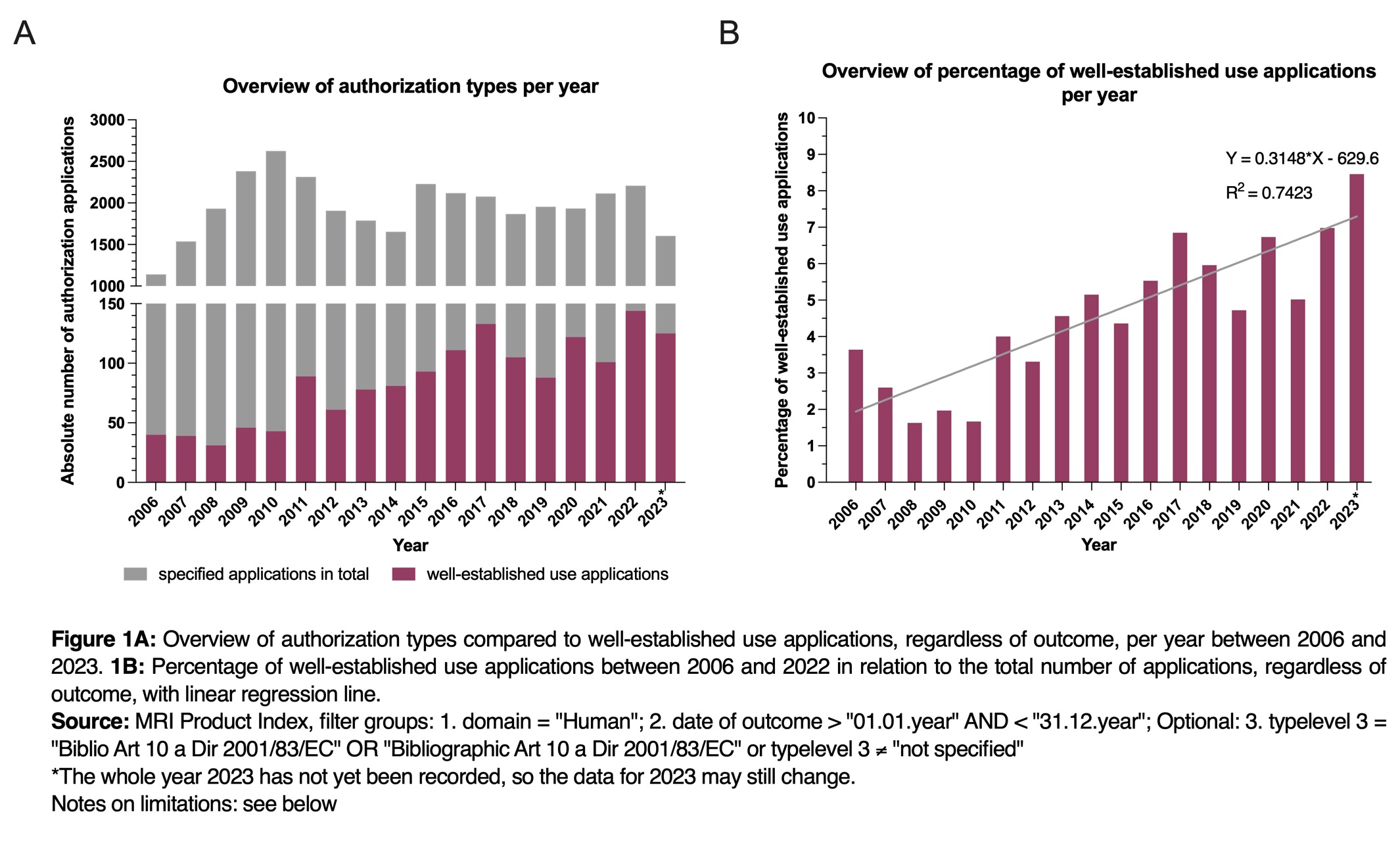
An analysis of the medicinal products listed in the European MRI Product Index whose marketing authorizations were applied for in mutual recognition procedures (MRP) or in the decentralized procedure (DCP) shows that well-established use marketing authorization applications are becoming increasingly popular among applicants (see Figure 1A).
From 2006 and 2023, the total number of admission applications varied, hitting a low of 1,100 applications in 2006 and reaching a peak of 2,600 applications in 2010. In case of well-established use applications, the numbers fluctuated between 30 applications in 2008 and 140 applications in 2022, showing an upward trend.
The average number of bibliographic applications in relation to the total number of applications for admission rose by approx. 5.4% between 2006 and 2023, showing a clear upward trend (see Figure 1B).
However, the reasons behind the growing trend in well-established use applications can only be speculated upon. It is likely that the industry‘s heightened interest in well-established use applications stems from their quicker and more cost-effective nature, bypassing the need for clinical and preclinical studies.
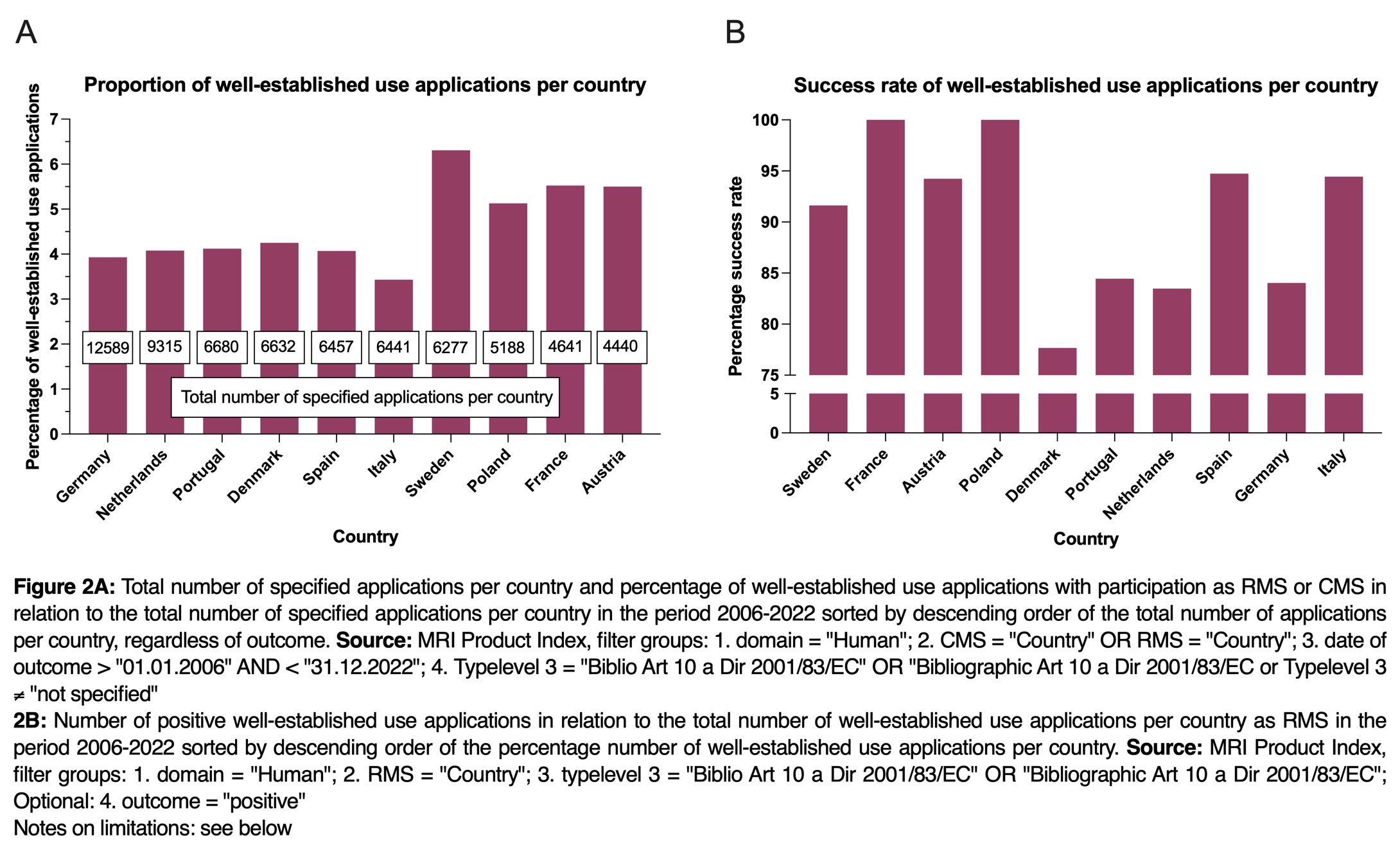
As shown in Figure 2A, the individual European countries are involved in well-established use approval procedures as RMS or CMS to varying degrees. Germany, for example, submitted the most applications in the period from 2006 to 2022, with more than 12,500 applications. This is followed by the Netherlands, with more than 9,000 applications, followed by Portugal, Denmark, Spain, Italy and Sweden, each with more than 6,000 applications, and Poland, France and Austria with around 4,000 to 5,000 applications.
In these countries, where many applications for approval are submitted, conditions appear to exist that provide an incentive for applicants, possibly through lucrative markets or easy approval procedures.
When examining well-established use applications across various European countries (both RMS and CMS) with with a comined total of over 4,000 applications from 2006 to 2022, notable variations emerge concerning the number of well-established use applications in comparison to the total number of applications in each country, as illustrated in Figure 2A. Notably, Sweden leads with the highest proportion well-established applications, accounting for approximately 6 %. In contrast, Italy lags at the lower end of the spectrum with around 3.5 % of well-established use applications. Germany, with just under 4 %, also falls towards the lower end of the scale. One potential explanation of the comparatively low percentage of well-established use applications in Germany could attributed to the stringent assessment conducted by the German authorities.
This is because companies, when selecting the RMS for well-established use applications, carefully consider their prospects of successful approval. Examining the percentage of successful authorizations in EU countries with over 4,000 authorization applications from 2006 to 2022 reveals distinct variations in success rates (refer to Figure 2B). Notably, only France and Poland have a 100% success rate for well-established use applications. In contrast, Denmark exhibits the lowest success rate at just below 80 %. Germany, with a success rate slightly below 85%, falls within the lower midrange.
Considering the data, France and Poland emerge as the most viable choices for RMSs. These countries exhibit extensive experience with the process, having submitted over 250 well-established use admission applications since 2006, coupled with a remarkable 100% success rate. However, it is crucial to emphasize that the selection of RMS should always be evaluated on a case-by-case basis. The authorities in Poland or France should not be universally considered as standard recommendations.
When interpreting the results, it is important to note that a 100% success rate does not necessarily indicate that the country as an RMS does not pose any challenges. The success rate of well-established use applications may be influenced significantly by efficient review procedures, transparent communication with the applicant, as well as expertise, and potentially the capacity of the authority or the location of the applicant.
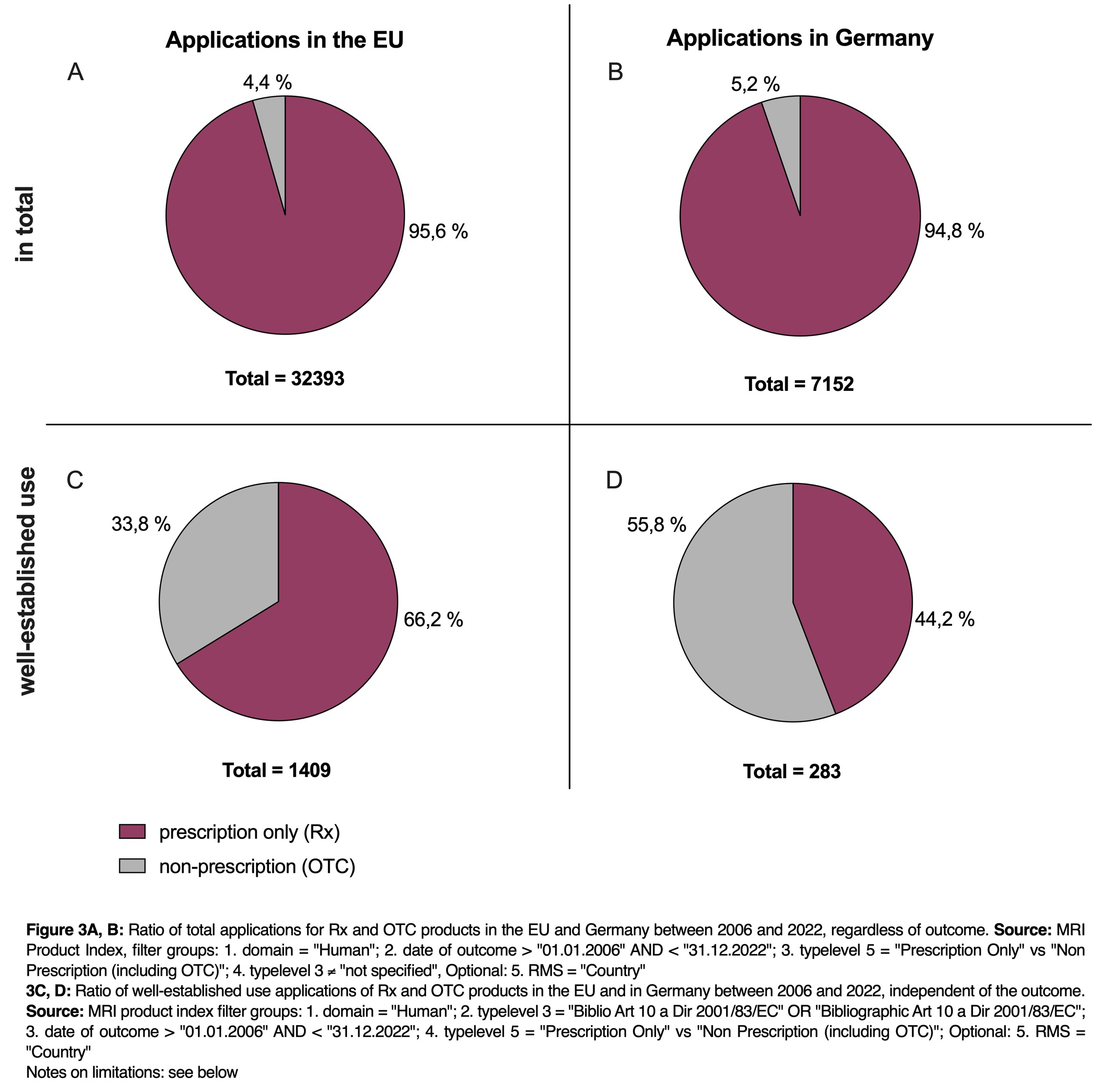
When comparing all marketing authorization applications for prescription (Rx) and non-prescription (OTC) medicinal products between 2006 and 2022 in the EU only 4.4 % of the more than 32,000 applications were submitted for OTC products (see Figure 3A). This trend is consistent when considering marketing authorization applications submitted in Germany in isolation, where the ratio is comparable at 5.2 % for OTC products (refer to Figure 3B).
Examining the well-established use authorization applications for both prescription-only and non-prescription medicinal products in the EU from 2006 to 2022 reveals that twice as many applications, accounting for 66.2 % of total applications, were submitted for prescription-only medicinal products compared to non-prescription ones (refer to Figure 3C).
If Germany acts as the RMS for the well-established use authorization application, a different picture emerges (see Figure 3D). With Germany as the RMS, significantly more well-established use applications are submitted for OTC products (55.8 %) than the average for all RMSs in the EU (33.8 %).
In Germany, there is a a substantial market for alternative medicine, with a distinct emphasis on OTC product. Companies show a keen interest in introducing OTC products to German market, and a well-established use marketing authorization application appears to be a more fitting choice when compared to other application types. This preference likely stems from the comprehensive literature available for OTC products, facilitating the straightforward demonstration of their general medical use which is required for a well-established use marketing authorization application. Additionally, authorities may also find it easier to assess the safety profile of OTC products.
Although the phrase "well-established use marketing authorization application" may initially be associated with dusty books, it is gaining popularity as an increasing number of medicinal product secure approval through this procedual approach. The increasing popularity and success of well-established use applications suggest that they should be considered not only as a complement but as a valuable alternative to traditional marketing authorization procedures, potentially paving the way for faster availability of safe and effective therapies in medicine.
We too witnessing an increase in well-established use approval procedures in our day-to-day operations. As a consulting service provider to the pharmaceutical industry, we are receiving more and more requests for well-stablished use authorization procedures.
Nevertheless, authorities express scepticism towards well-established use applications. The minutes from the meeting of 18-20 July 2023 by the Coordination Group for Mutual Recognition and Decentralized Procedures - Human (CMDh) detail a stronf recommendation for applicants to refrain from submitting well-established use application when a reference medicinal product is available on the market. In such instances, it is advised to submit generic applications.
An amendment is also to be integrated into the EU pharmaceutical package, which states that in future a well-established use application should only be submitted if no reference medicinal product for the same active pharmaceutical ingredient is or has been authorized within the EU for the same therapeutic use and for the same route of administration. Otherwise, a generic or hybrid application must be submitted. Therefore, even if a marketing authorization no longer exists in an EU country, this would prevent a well-established use application. However, it is evident that the negotiations are poised to be extensive and challenging, due to the comprehensive nature of the proposed legislation. The EU Parliament will also be newly elected in spring 2024. It is therefore unlikely that the lae will come into force in the next two years. This is positive news for planned and upcoming well-established use authorization procedures with a reference medicinal product authorized in the EU.
These new stringent regulations would make what is and efficient, fast and cost-effective process of well-established use approval much more difficult. Well-established use authorizations may not be abolished completely however, the new criteria would be significantly tightened which could slow down the upward trend seen for well-established use approval applications.
To ensure the success of a well-established use authorization application, it is highly recommended to carefully consider the requirements, possibilities, hurdles, strategies and chances of success. We generally recommend agreeing the procedure and the content of the documentation with the relevant authorities in advance as part of a scientific advice process.
Notes On Limitations: