Wir sind am 01.04. umgezogen! Unsere neue Adresse ist: Reimerstwiete 11, 20457 Hamburg
What GDP non-compliance reports reveal about structural weaknesses.
There are currently 50 publicly accessible GDP non-compliance reports listed in the EudraGMDP database: A clear indication that violations of Good Distribution Practice (GDP) requirements are not isolated cases. They often reveal fundamental structural weaknesses in organization, responsibility and implementation within pharmaceutical wholesale companies.
It is therefore worth taking a closer look. What recurring patterns can be identified? What conclusions can be drawn for your own preparation for future inspections?
We have analyzed the latest non-compliance reports and summarized the most important findings.
What is the EudraGMDP?
Since its launch in April 2007, the EudraGMDP database has been the central European directory for GMP and GDP certificates as well as for non-compliance reports. It is operated by the European Medicines Agency (EMA) and serves the competent authorities of the EU member states as an essential tool for monitoring medicinal products. Originally designed exclusively for GMP data, the database was expanded to include GDP-relevant content in 2014 as part of the implementation of the Falsified Medicines Directive 2011/62/EU. This also includes GDP non-compliance reports, i.e. public reports on serious breaches of good distribution practice.
Despite the common European framework, the technical connection of the national inspection and approval systems to EudraGMDP was by no means uniform. Many countries, including Spain with its Labofar system, worked with independent national platforms. These initially had to be connected to the EMA infrastructure at great expense, which made the data flow more difficult or delayed in the initial phase. In Germany, for example, PharmNet.Bund handles the interface. Here, the BfArM transmits verified GDP and GMP documents to the EMA every working day [1].
France, on the other hand, is one of the countries that was comparatively late to adopt the digital data flow. Nine years after the database was launched, GMP certificates were still only issued in paper form. It was not until April 15, 2016 that the French medicines authority ANSM began publishing GMP certificates in EudraGMDP [2]. However, this delayed integration marked the beginning of France's stronger commitment to digital transparency in the regulatory environment.
A milestone in the technical development of the database was the introduction of the SPOR framework (Substance, Product, Organization and Referential master data). In particular, the integration of the Organization Management Service (OMS) in January 2022 brought about a far-reaching change: since then, national authorities have only been able to issue certificates or non-compliance notifications if the company concerned is registered in the OMS with correct master data. This measure promotes standardization and interoperability within the EU approval system and ensures that all data can be referenced consistently and unambiguously.
What must be reported?
The reporting obligations in EudraGMDP are defined by several EU legal acts, namely Directive 2001/83/EC for medicinal products for human use, Directive 2001/82/EC for veterinary medicinal products and the Falsified Medicines Directive 2011/62/EU. They oblige national authorities to provide all relevant information on:
- Manufacturing and wholesale licenses,
- GMP and GDP certificates,
- Registrations of active ingredient manufacturers,
- as well as confirmations of GMP or GDP non-conformity
in the central EU database.
This is made particularly clear in Article 111(7) of Directive 2001/83/EC, which states:
"[if] the inspected entity does not comply with the legal requirements and/or the principles and guidelines of Union law on good manufacturing practice or good distribution practice, this information shall be registered in the Union database."
When looking at the country distribution of the reports viewed, it becomes clear that, despite this obligation, no GDP non-compliance report from France is currently available (see Figure 1). Among the reporting countries, Germany (24%), the Czech Republic (22%) and Romania (16%) represent all reports.
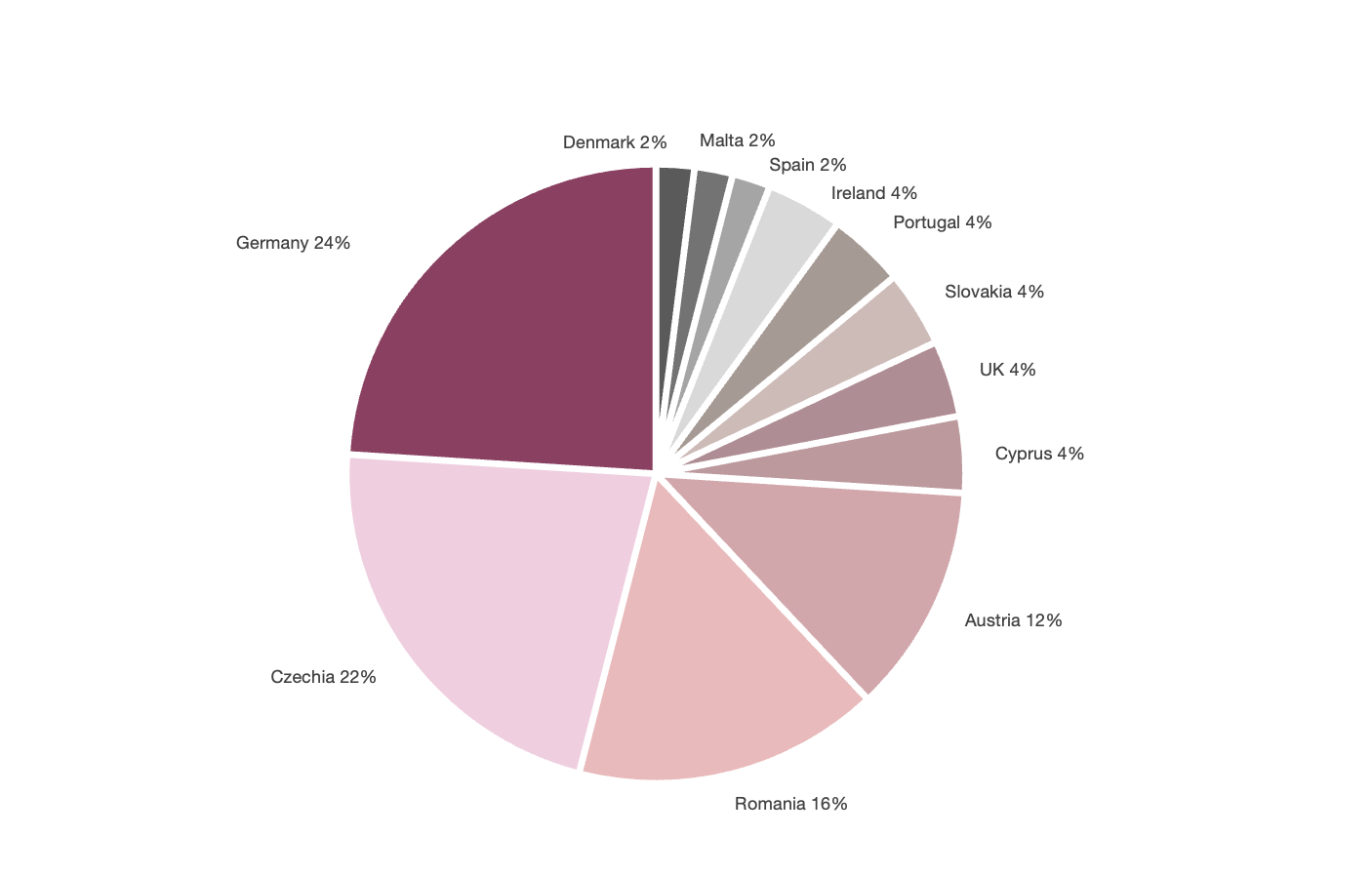
How can this discrepancy be explained? As some of the competent national authorities do not comply with the transmission of these reports, it is only possible to speculate. The list of competent national authorities in the European Economic Area shows that Germany has a particularly dense network with a total of 47 competent surveillance authorities and thus the basis for a well-established and regular inspection practice [4]. The high number of reporting authorities could therefore have led to a comparatively high number of published non-compliance reports in Germany. However, this consideration cannot be applied to other member states, which generally only have one to three competent authorities [4]. Nevertheless, it is worth taking a closer look at the shortcomings observed.
What can we learn from these reports?
Our analysis shows that it is not individual errors, but structural weaknesses that are repeatedly noticeable. For this purpose, we evaluated all available reports with regard to their listed deficiencies and assigned them to the corresponding chapters of the GDP guideline, whereby several deficiencies were usually assigned to one report. This allowed us to aggregate deficiencies and cluster them thematically.
The following chart shows the distribution of reported deficiencies across the chapters of the GDP guideline. The area of operations is by far the most affected, followed by personnel and quality management. It is particularly striking that within the personnel chapter, most of the complaints are directly linked to a central key role, the person responsible.
.png)
A closer look shows that in more than three quarters of the cases complained about, the responsible person was either not available, not permanently appointed or did not fulfill their role in accordance with the legal requirements (Figure 3). This function is mandatory for every pharmaceutical wholesaler, which is regulated in Germany by §52a AMG.
Many of these complaints could be avoided through clear responsibilities, active integration into the quality system and appropriate qualifications of the people involved. A regular comparison of the existing structures with the regulatory requirements is therefore not only legally required, but also crucial for a successful pharmaceutical wholesale business.
.png)
A first look - with potential for more
With our evaluation, we were able to gain an initial insight into which GDP chapters are particularly susceptible to official complaints in practice. The database therefore not only offers transparency, but also the opportunity to learn from the mistakes of others. We will continue to follow developments closely and thus build a bridge between regulatory theory and operational reality.
In this way, we can learn together from specific cases and not just manage GDP compliance, but actively shape it.
In recent years, we have successfully assisted numerous companies with precisely these challenges: be it in the appointment of a responsible person, the establishment and further development of quality systems, preparation for inspections or the practical training of personnel.
Are you also facing this challenge? Then get in touch with us!
References