Wir sind am 01.04. umgezogen! Unsere neue Adresse ist: Reimerstwiete 11, 20457 Hamburg
An analysis of EMA approval data from key procedures for antineoplastic drugs.
Drug research is constantly changing, characterized by new scientific findings and regulatory requirements. After Overview of central approval procedures From the past year, this article shows current trends and strategic developments in this dynamic area.
The analysis of the centrally submitted applications for approval showed stable development with an average of just over 80 new registrations per year in the period 2013 to 2023. The consistently high rate of new registrations was also confirmed last year with 114 positive opinions and 80 market approvals [1]. Oncology is particularly dynamic: Looking at the period from 2018 to 2024, around every fifth central approval was attributable to an oncological drug. The medical relevance is undisputed; cancer remains one of the most common causes of death worldwide. The increasing incidence of oncological diseases will increase the need for innovative treatments and solution-oriented regulatory strategies. It is estimated that the number of new cases will rise from around 20 million annually to up to 32 million by 2050 [2].
A look at the distribution of cancers shows that just under half of all new cases are due to breast cancers and cancers of the colon, lung and prostate (Figure 1). While the 5-year survival rates for breast and prostate cancers are high at over 80%, they are only 22% for lung cancer. The calculated coefficient of medical need In comparison, this disease reaches the highest value. It is calculated by multiplying the relative 5-year survival rate by the proportion of new cases of cancer. With regard to the survival rate for bronchial carcinomas in the 2000s, a relative increase of 48% is already showing a positive development [4]. This is not least due to the proportion of antineoplastic research, which is also evident in IQVIA evaluations. In the past year, 25% of clinical trials were dedicated to the development of new oncological agents [2].
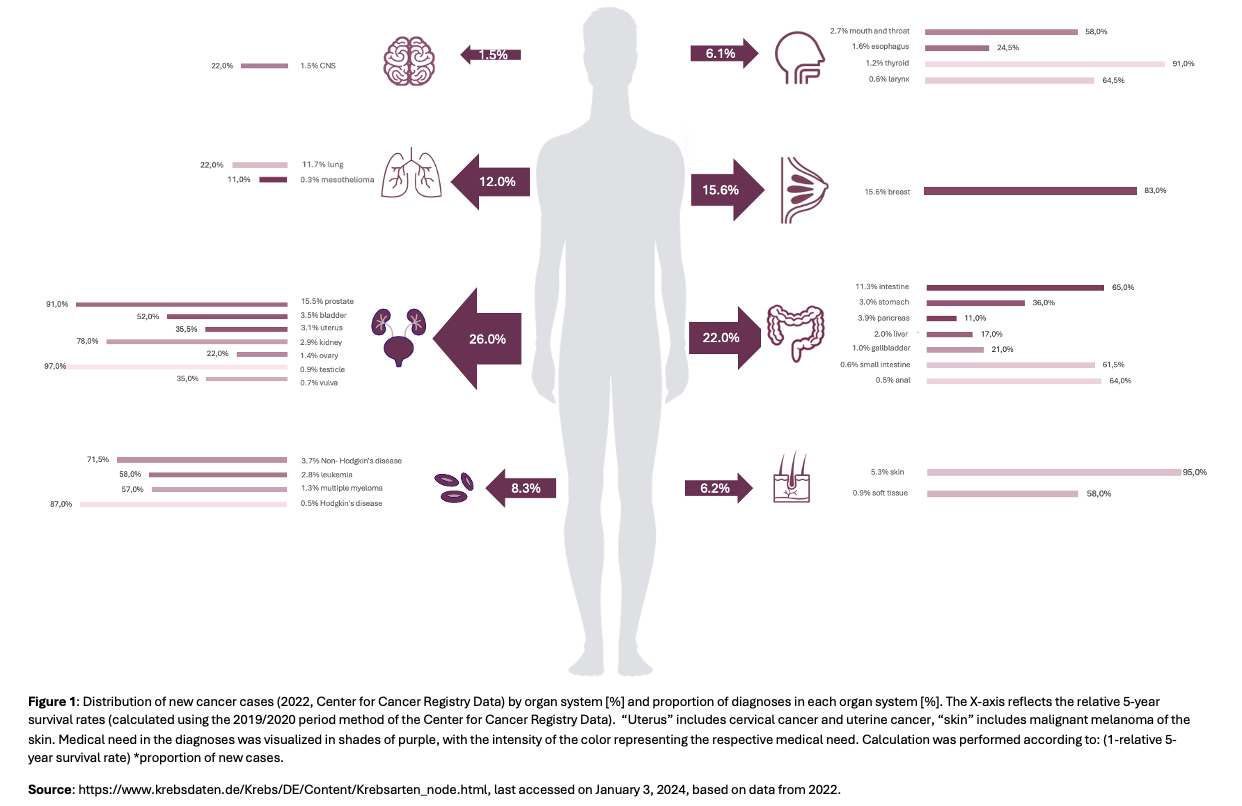
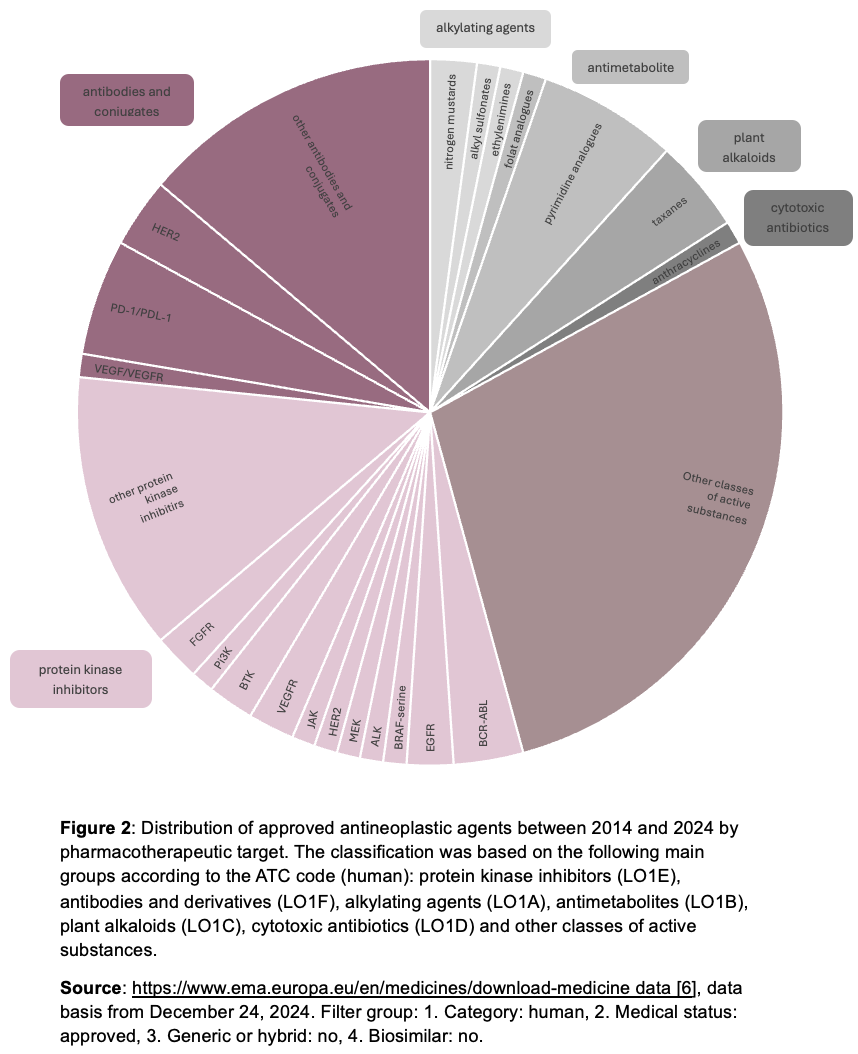
While treatment options have been exhausted for decades in surgery (s), chemotherapy or radiation (s), drug development today is increasingly focused on targeted therapeutic approaches. As part of this target therapies tumor-specific metabolic pathways are addressed, including specific enzyme inhibitors or antibody derivatives. Immunomodulatory agents are also used to modulate growth processes or to induce apoptotic processes in cancer cells. This targeted specificity on tumour tissue helps to reduce side effects and increases therapeutic effectiveness. It is therefore not surprising that the proportion of antibody derivatives and protein kinase inhibitors has increased significantly over the last ten years, while approvals of antimetabolites and alkylating agents have declined (Figure 2). In addition, combination therapies and approaches to enhance effects are becoming increasingly important. The already mentioned low survival rates of aggressive bronchial cancer can be explained primarily by the development of resistances following initial treatment. Strategies that minimize possible development of resistance are therefore decisive for treatment success. Preliminary results of an open phase I study already suggest that the effectiveness of a PD-1 checkpoint inhibitor can be increased in bronchial carcinomas by combining it with special bacteria that settle in the intestine as “Live Biotherapeutic Products” (LBP). Tumor vaccinations, most recently in combination with checkpoint inhibitors, are also the subject of current research.
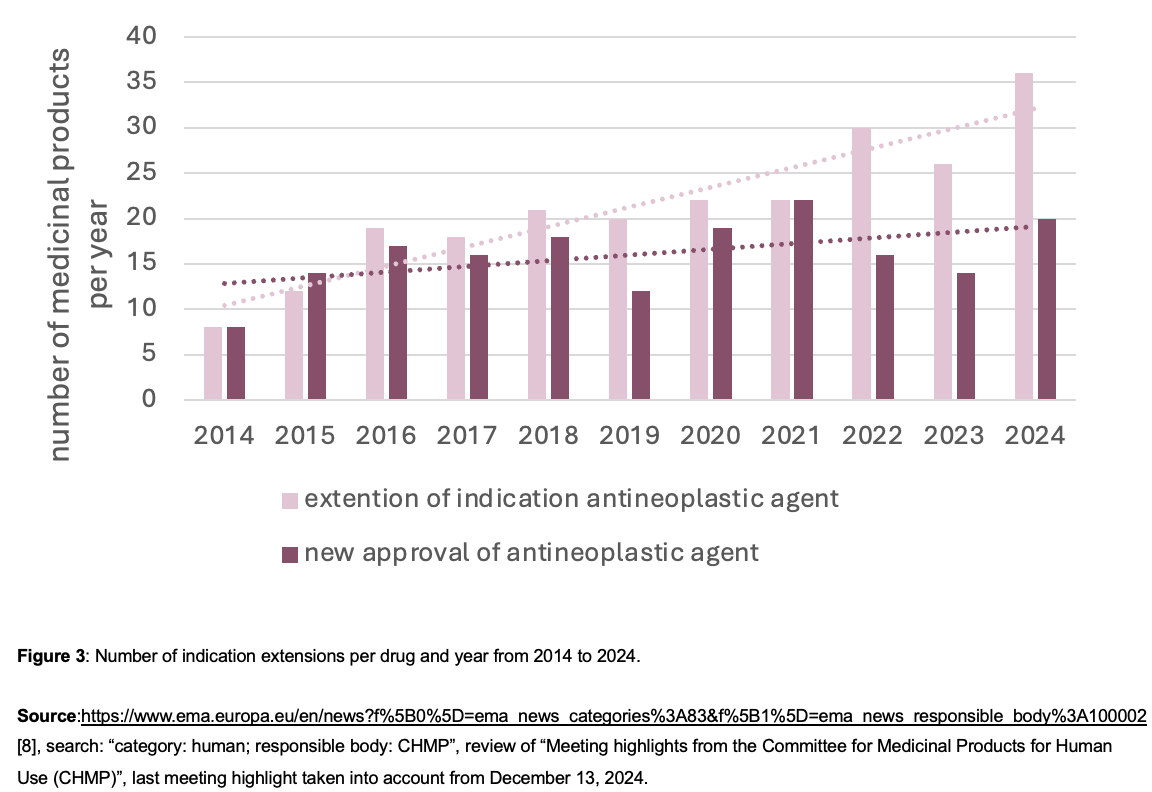
Another trend in oncological drug development is the expansion of indications. Compared to 32 proposed indications extensions in 2014, these have more than doubled in the last ten years. While the number of new registrations and indication extensions was still the same in 2014, in 2024, 80% more indication extensions were applied for compared to the new antineoplastic registrations this year (Figure 4).
The strategic expansion of therapeutic benefits is a frequently used adaptive approach to close treatment gaps. It makes it possible to specifically expand the potential of an already approved drug so that access to new patient groups can be expanded and the market position can be strengthened. In addition to data from controlled clinical trials, there is an increasing number of Real World Data (RWD) in focus. These can not only contribute to the identification of suitable subgroups, but also as supporting population representatives Real World Evidence (RWE) can be used in approval procedures, which, last but not least, can save financial resources. With 111 new registrations by the EMA in 2018 and 2019, all included data from RWE [7]. While they are already being used as part of post-market surveillance, they are now also increasingly being used in the area of drug development. During this period, antineoplastic drugs examined relied on RWE for clinical development in 50% of cases [7]. The EMA's Scientific Explorer published last year as an AI-supporting tool for European regulatory authorities and the guidelines for using RWE published in the same year confirm the potential. The increasing use of RWD/RWE, particularly in light of the implementation of artificial intelligence, requires a carefully coordinated strategy that combines medical evidence with regulatory and economic frameworks for successful approval.
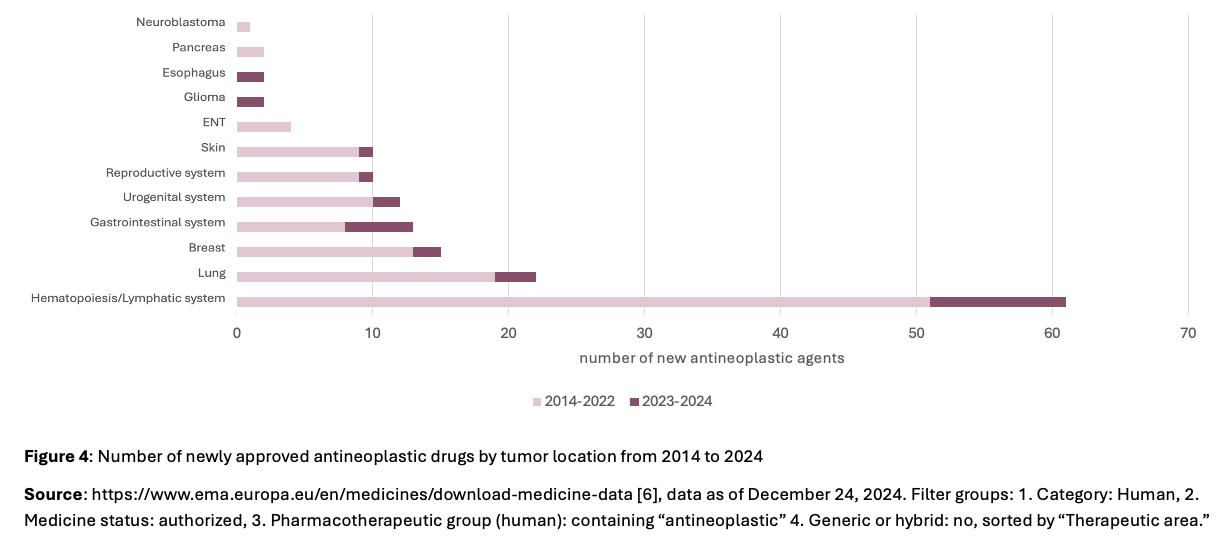
The already mentioned use of Real World Data can specifically complement the complex interplay of key factors in drug development, such as the presence of addressable targets, market potential and medical needs (comparison with Figure 1), and contribute to the successful development of new therapeutic approaches. In the past two years, there have been new registrations in two diseases with a high medical need, which had no significant changes in death rates for over 20 years [4]. In the area of esophageal carcinomas and gliomas, one new active ingredient has been approved in the last two years (Figure 4), supported, among other things, by findings from Real World Data to identify subgroups and addressable target structures.
The continuing high demand for treatment in oncology and the rapid development of new drugs suggest that this area will continue to be the focus of research in the future. In the future, the variety of treatment options will enable patients to be treated even more precisely and individually in order to meet the challenges of this diverse disease.
testimonials
Notes on limitations: